Weight-to-weight medicine may get a new FDA approval in six months in Vegovi
For Vegovi, the Blockbuster Weight Loss Drug of Patel Protection-Novo Nordks, which has a GLP-1 active ingredient and at least twice as effective-is expected to expire by the end of the end.
Michael Silk | UCS | Getty
Novo Nordis On Thursday, his blockbuster weight-Las drug Vegovi could get extended approval from the US Food and Drug Administration in six months.
Chief Financial Officer Karston Munk Nudson told CNBC that Danish Pharmaceutical Company has received a preference review in the application for approval of Vegovi as a treatment to reduce the risk of cardiovascular and cardiovascular disease.
Greenlight of the Health Agency can boost the possibility of insurance coverage of the highly demanded drug.
“I will say today, (the result will take place in less than six months),” Noodson told CNBC’s Juliana Tatalbom on “Street Signs”.
Earlier on Thursday, Novo Nordisk announced a plan to get an extended approval from the FDA in the third Third quarter, but did not share any timeline. It recorded a record profit and sales in the back of the success of obesity.
Increasing health app
Late-page test data in August showed Vegvi Reduced the risk Among the major cardiovascular and cardiovascular events such as a heart attack or stroke compared to placebo.
“Selective studies, in obese people with established cardiovascular and cardiovascular diseases, reduce the risk of cardiovascular and cardiovascular? And the answer is, yes it is 20%,” Noodson said on Thursday.
The results of the close of the necklaceSelect“The test was seen as a boon as an ambition to go beyond the image of Novo Nordisk’s Wagoi”Vanity drugs“
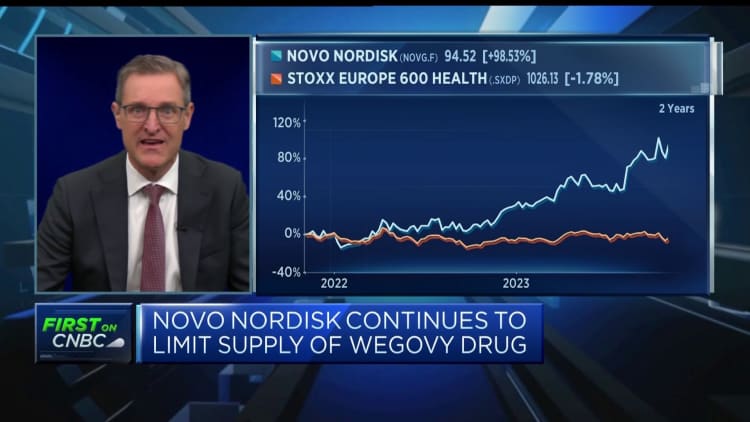
These findings have given additional boost of the company’s stock, which is on UP this year. The shares were 1.5% higher on Thursday morning after his revenue report; To date, the shares are almost 45% higher in the year.
Nudson said the company would submit a detailed copy of the test findings in approximately 10 days, after which the FDA will have six months to give its results.
He further said that the case of drug use will expand and the insurance companies will increase the chances of paying for treatment. Some insurers refrain from covering the drug so far-which the United States list is $ 1,350 a month-with aimed at losing weight.
“The cost of products is also reflected in the values they have given to the community,” Noodson said.
“So more of the data we are able to produce, whether it is on cardiovascular disease or acute kidney disease or other commercials, when we discuss with the payers and insured are part of the story.”
However, with approval, the lack of supply can obstruct the rollout of the drug. Nudson said on Thursday that the company was increasing production.
He said, “I can promise you that next year that we are scaing significantly by the Vegvi supply.”












Post Comment